In a recent study published in the British Journal of Dermatology, it was found that for patients with moderate psoriasis, treatment with risankizumab demonstrated superior efficacy compared to apremilast.
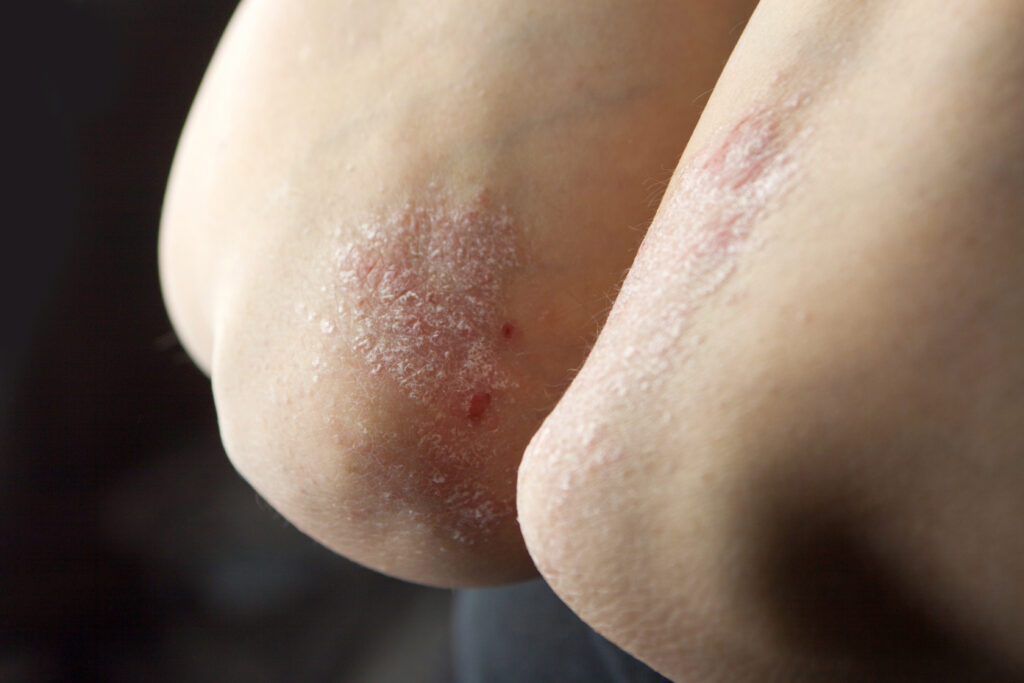
AbbVie’s phase 4 head-to-head study, IMMpulse, evaluated both risankizumab, an interleukin-23 inhibitor, and apremilast, which inhibits phosphodiesterase 4.
The study was a multicenter, randomized, open-label, efficacy assessor-blinded study which took place over the course of 52 weeks. It included a total of 352 patients over 18 years with a diagnosis of moderate chronic plaque psoriasis who were candidates for systemic therapy. Of these, 118 received subcutaneous risankizumab at a dosage of 150 mg at week zero and week four. A total of 235 received oral apremilast at a dosage of 30 mg twice a day.
At week 16, if those patients receiving apremilast had not achieved a Psoriasis Area and Severity Index (PASI) 75 response, they were re-randomized to receive either risankizumab or apremilast.
At week 16, results showed that 55.9% of patients treated with risankizumab achieved the co-primary endpoint of PASI 90 compared to only 5.1% of those treated with apremilast.
As for the co-primary endpoint of static Physician’s Global Assessment (sPGA) 0/1, this was achieved by 75.4% of those treated with risankizumab compared to 18.4% of apremilast-treated patients.
A total of 83 apremilast-treated patients who failed to reach PASI 75 by week 16 were switched to risankizumab and 78 continued with apremilast. By week 52, 72.3% of these patients who had switched to risankizumab had achieved PASI 90 compared to only 2.6% who continued on apremilast.
Of those patients who received continuous treatment, results at 52 weeks demonstrated that 73.7% of those treated with risankizumab achieved PASI 90, and 63.6% achieved PASI 100. For those treated with apremilast, 4.5% achieved PASI 90 by week 52 and 2.7% PASI 100.
Patients were also provided with a Treatment Satisfaction Questionnaire for Medication. At week 16, those treated with risankizumab reported higher overall scores than those treated with apremilast:
Risankizumab | Apremilast | |
|---|---|---|
Satisfaction with Effectiveness | 80.6 | 46.9 |
Satisfaction with Convenience | 84.9 | 69 |
Global Satisfaction | 86.2 | 47.7 |
The most frequent adverse events reported in risankizumab were COVID-19 and nasopharyngitis. For those receiving apremilast, diarrhea, nausea, and headaches were most common.
The study concluded that “risankizumab treatment can significantly improve clinical outcomes in systemic-eligible patients with moderate psoriasis compared to apremilast.”
















