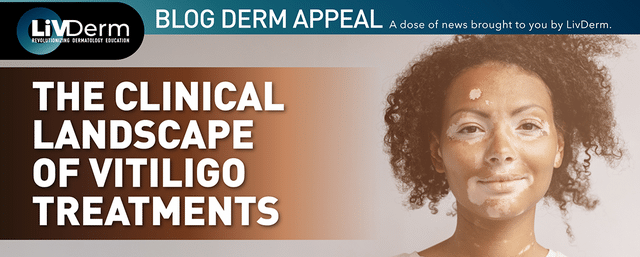
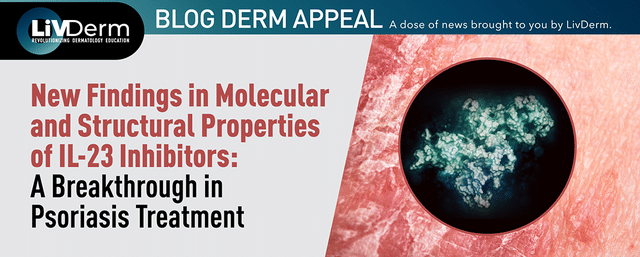
Galderma announced positive results from its pivotal phase 3 Olympia 2 trial at the 25th World Congress of Dermatology.
This 16-week study evaluated the safety and efficacy of nemolizumab in adult patients with moderate-to-severe prurigo nodularis.
Nemolizumab is a first-in-class investigational drug targeting interleukin (IL)-31 signaling. It is widely recognized as the itch cytokine and a central mediator of inflammation and fibrosis in prurigo nodularis.
During the study, patients were randomly assigned to receive either nemolizumab or placebo. Patients receiving nemolizumab and who weighed less than 90 kg were given a loading dose of 60 mg followed by 30 mg every four weeks. Those weighing 90 kg or more received 60 mg every four weeks.
Results showed that a total of 19.7% of patients who were treated with nemolizumab achieved an itch-free state by week four compared with only 2.2% of those treated with placebo.
By week 16, 56.3% of nemolizumab-treated patients had achieved a greater than or equal to a four-point improvement from baseline on the peak pruritus numerical rating scale. This was in comparison to 20.9% of those who received placebo.
The study met all primary and secondary endpoints and demonstrated that nemolizumab improved not only itch but also skin clearance and sleep disturbance.
“The itch associated with prurigo nodularis is uniquely intense, causing significant sleep disturbance that substantially reduces quality of life. These data demonstrate once again the massive burden those with prurigo nodularis face, and the extent of nemolizumab’s potential to address it.”
Baldo Scassellati Sforzolini, MD, PhD, MBA, global head of research and development at Galderma