Arcutis Biotherapeutics recently announced that the U.S. Food and Drug Administration (FDA) approved its supplemental new drug application (sNDA) for the expanded indication of ZORYVE (roflumilast) cream 0.3% for the topical treatment of plaque psoriasis in children 6 to 11 years of age.
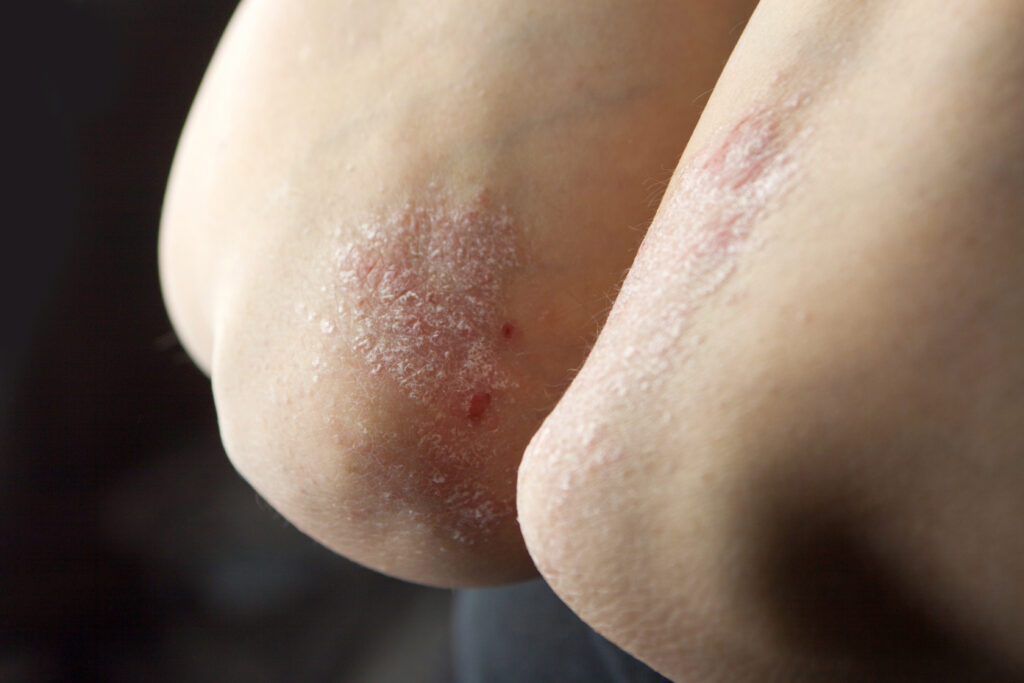
Roflumilast is a once-daily, steroid-free, topical cream that can be applied to intertriginous areas (skin folds). It is the only topical treatment for which efficacy in intertriginous areas was evaluated in pivotal trials and currently, the only product specifically indicated for use in these sensitive areas.
According to Adelaide A. Hebert, MD, professor and chief of pediatric dermatology at McGovern Medical School at UTHealth Houston and Children’s Memorial Hermann, and LiVDerm faculty member, “In children, psoriasis ranges from mild to severe, and more often appears on sensitive areas including the face and skin folds, compared to adults. Topical steroids are commonly recommended medications for the treatment of pediatric psoriasis. However, they come with safety and tolerability concerns related to long-term use. Steroid-free topical treatments that can be used on sensitive areas are especially needed for managing plaque psoriasis in younger children.”
The approval of the expanded indication is based on the data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children aged 6 to 11 years with plaque psoriasis. The study revealed that the pharmacokinetic, safety, tolerability, and efficacy data were generally consistent with the data that was derived from the DERMIS-1 and DERMIS-2 pivotal Phase 3 trials that were conducted in adults.
According to the press release from Arcutis Biotherapeutics, the results from a second MUSE study in children aged 2 to 5 years will be the subject of a future FDA review. This is also true for an ongoing open-label extension study that aims to assess the long-term safety of roflumilast cream 0.3% in individuals with plaque psoriasis aged two years and older.
“Young children with plaque psoriasis lack treatment options, which is why today’s decision by the FDA represents a meaningful advancement for this pediatric population, their parents, and caregivers. ZORYVE has been shown to be safe, well tolerated, and effective; all critical factors for treating children with plaque psoriasis. We are excited to now be able to expand the availability of ZORYVE to children as young as 6 years old, offering them and their families an important new steroid-free treatment option to consider along with their healthcare providers.”
Patrick Burnett, MD, PhD, FAAD, chief medical officer at Arcutis
Head over to our Psoriasis Digital Education Hub to access complimentary CME activities, podcasts, guidelines, and more. You can also tune in to Dr. Hebert’s podcast, Clinical Pearls About Treatment of Psoriasis.
Don’t forget to also enroll in our MOPD Foundations of Pediatric Dermatology Certificate Course, our comprehensive six-module program designed to build your foundational knowledge of some of the most common skin conditions affecting the pediatric population.

















