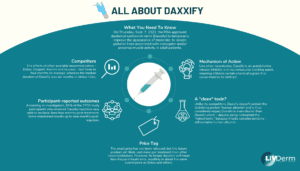
All About Daxxify
In greatly anticipated news from the U.S. Food and Drug Administration (FDA), the novel botulinum toxin injectable daxibotulinumtoxinA-lanm (Daxxify) has just been given the agency’s green light. Pivotal studies show the drug can temporarily improve moderate-to-severe frown lines in adults for a six-month duration, which is significantly longer than the three- to four-month treatment results for Botox and other major competitors.
Developed by manufacturer Revance Therapeutics, the FDA approval of Daxxify was based on data from more than 2,700 patients and approximately 4,200 treatments. The company reported that 98% of trial participants had “no or mild wrinkles four weeks post-injection.” The median duration lasted six months, and some patients maintained treatment results for as long as nine months.
Download the helpful infographic below and share it with your patients to answer any questions they might have. Your practice likely doesn’t carry the product yet, but many clinics are beginning waitlists for this in-demand treatment. The good news is that injection techniques will be similar to the neuromodulators you already offer, so compiling a list of interested patients NOW will allow you to hit the ground running with this treatment as soon as it’s available to you!