As headlines report the recent revelation of benzene, a known human carcinogen, in acne products, a profound wave of concern has gripped both healthcare professionals and consumers. This resurgence of the dangers of benzene in consumer-facing products has prompted requests for a critical reevaluation of existing protocols whilst emphasizing the urgent need for transparency, accountability, and rigorous safety measures. As we learn more about these latest developments, it is necessary to examine past instances of benzene contamination to grasp the full extent of the implications stemming from this new discovery.
Moreover, given the widespread prevalence of acne as one of the most common skin conditions in the U.S., it is imperative that consumers are informed about the associated risks and urged to discuss these findings with their dermatologist.
Valisure Reports: Benzene Found in Acne Products
The latest findings, originating from an independent testing laboratory, Valisure, have brought alarming revelations to light. In their comprehensive study, Valisure evaluated 66 over-the-counter (OTC) and prescription-based acne products containing benzoyl peroxide (BPO). Their analysis revealed elevated levels of benzene within these products, some at higher levels than others.
As a result of their findings, Valisure filed a Citizen’s Petition with the FDA, requesting an immediate investigation and the removal of these BPO-containing products from the market.
This petition reiterates the fact that the Centers for Disease Control and Prevention and the Department of Health and Human Services have determined that benzene causes cancer in humans, adding that the World Health Organization and the International Agency for Research on Cancer have both classified benzene as a Group 1 compound, therefore being carcinogenic to humans.
The U.S. Food and Drug Administration (FDA) also recognizes benzene as a Class 1 solvent, stating it should not be used in the manufacture of drug substances, excipients, and drug products because of its unacceptable toxicity. They do, however, allow for a maximum of 2 parts per million (ppm) if their use is unavoidable in order to produce a drug product with a significant therapeutic advance.
According to the petition, “Considering the long history and widespread use of BPO products, it does not appear that they currently constitute a significant therapeutic advance; therefore, any significant detection of benzene should be deemed unacceptable.”
Valisure’s investigation unveiled benzene concentrations up to 12 times the allowed amount, with levels increasing significantly when subjected to higher temperatures, tests which were done in order to replicate how the products can break down over time. These findings only served to intensify potential health hazards.
Even more disturbing is that the presence of elevated benzene levels extended beyond the confines of the BPO-based products, being found to emanate externally into the surrounding air, thereby posing an additional risk of inhalation exposure. There is significant concern surrounding this point as “levels detected within a product may only be a fraction of the total production of benzene and potential exposure to a patient.”
What the Experts are Saying
Following these disturbing findings, renowned dermatologist and LiVDerm Advisory Board Medical Chair, Christopher G. Bunick, MD, PhD, has weighed in on the topic.
“Dermatologists are waking up this morning to the shocking news that the widely-used over-the-counter and prescription medicine, benzoyl peroxide, decomposes into the well-known carcinogen benzene. Benzene contamination has plagued numerous skin care products recently, but this time it is different. Here, it is not contamination, rather benzoyl peroxide breaks down into benzene, stimulated to do so by the formulation of the products themselves. What is concerning is that nearly all benzoyl peroxide-containing products tested formed benzene (even the new triple combination topical), often at levels 2 to 1000X higher than the emergency/conditional FDA limit of 2 ppm.”
In reference to the increased levels at higher temperatures, Dr. Bunick states, “To make matters worse, benzoyl peroxide breakdown into benzene was accelerated by exposure to elevated temperatures equivalent to a hot bathroom or car, meaning that patients and consumers must be mindful of how to store their benzoyl peroxide-containing product (not in a car!). Even more concerning, as benzoyl peroxide breaks down into benzene, the gaseous form of benzene was found to penetrate through sealed packaging at levels nearly 1000X higher than the EPA lifetime exposure limit (0.4 ppb) for the increased risk of 1 cancer per 100,000 people. Thus, benzoyl peroxide-containing acne products unsuspectedly are also an inhalation risk.”
“It is important to note that the specific problem with benzene in benzoyl peroxide products does not appear to be a contamination issue from a specific ingredient, but instead the inherent instability of the benzoyl peroxide molecule that breaks down and forms benzene.”
David Light, co-founder and president of Valisure has stated “This discovery of benzoyl peroxide’s fundamental instability and formation of benzene is substantially different than Valisure’s previous findings of benzene in sunscreens, hand sanitizers, and other consumer products. The benzene we found in sunscreens and other consumer products were impurities that came from contaminated ingredients; however, the benzene in benzoyl peroxide products is coming from the benzoyl peroxide itself, sometimes at hundreds of times the conditional FDA limit. This means the problem broadly affects benzoyl peroxide products, both prescription and over-the-counter, and necessitates urgent action.”
Previous Instances of Benzene Contamination in Consumer Products
Over the last few years, there have been other instances that brought benzene into the spotlight for contaminating various consumer-facing products, including sunscreen, hand sanitizers, and body sprays.
In May 2021, the same company, Valisure, unveiled their discoveries regarding several sunscreen products. Upon conducting tests on 294 unique batches of sun care products from 69 different companies, they identified detectable levels of benzene in 27% of these samples, with some containing up to three times the FDA threshold of 2 ppm. In response, Valisure filed a Citizen’s Petition urging the FDA to recall the affected sunscreens and after-sun care products.
Referring to these findings, Dr. Bunick stated “There is not a safe level of benzene that can exist in sunscreen products. Even benzene at 0.1 ppm in a sunscreen could expose people to excessively high nanogram amounts of benzene.”
Prior to this, Valisure asked the FDA to recall several hand sanitizer brands after they were found to be contaminated with benzene.
In late 2021, Proctor and Gamble announced the recall of 32 of their dry shampoo and conditioner products after benzene was found in them. Later, in October 2022, Unilever voluntarily recalled 19 of its brands of dry shampoo due to the potentially elevated levels of benzene contained in them.
Speaking at the 2022 South Beach Symposium, Dr. Bunick addressed the issue of benzene contamination in skincare products in his lecture entitled, “Putting Skincare Products Under the Microscope.” In his talk, he referenced several studies, including one examining the relationship between benzene exposure and leukemia risk. The authors found that “Exposure intensity in the highest exposed job was strongly related to leukemia risk, with the increase starting at around 0.8-1.6 ppm and with those in the highest exposure category being nearly 20 times more likely to develop leukemia than those who were unexposed.” Putting this into perspective for the audience and using 1.6 ppm as a reference point, Dr. Bunick posed the question – “If the subjects in the study saw increased risk at just 0.8-1.6 ppm, what does that say about the sunscreens containing 2 ppm or above?”
In 2023, after the news of benzene-contaminated dry shampoos, Dr. Bunick returned to the SBS stage, this time to share a lecture entitled, “Update on Contamination in Skincare Products: Benzene Returns to the News.” During this talk, he spoke about the lack of quality control regulations in the production of these products and touched on how some of these aerosolized products linger in the air and fill a room, thereby exceeding the EPA threshold for increasing the risk of cancer.
“The complexity of the manufacturing process and supply chain is a root cause of this crisis. Dermatological products, ranging from over-the-counter cosmetics to advanced therapies, involve intricate manufacturing processes. However, the quality control measures implemented by manufacturers have proven inadequate, allowing benzene contamination to persist.”
Elevated Benzene Risks
The instability of the products and further risk of inhalation is an echo of what we are now hearing from Valisure about the elevated benzene levels found in the air surrounding incubated BPO-containing acne products.
According to their study, after an initial stability study, 50°C was chosen as a stability temperature for a broader study of the 66 BPO-containing products. They were incubated for 18 days at 50°C (considered a reasonable temperature for products to be exposed to) and samples were measured for benzene at Day 0, 4, 10, 14, and 18. The results demonstrated substantial instability of BPO and its propensity to form alarmingly high levels of benzene in only 18 days.
Further testing at 70°C (also not an unreasonable temperature for a product to be exposed to in distribution or consumer handling and can be likened to the temperature inside a hot car) revealed that “even the most stable of the 66 formulations tested still produce over 2 ppm of benzene when incubated at 70°C for 14 or 18 days”
These findings prompted further testing by Valisure using SIFT-MS technology where they evaluated benzene levels in the surrounding air of an incubating BPO product.
Results from this analysis are particularly relevant for 70°C as it is “within the known temperature range of a hot car, and 40°C is within the known temperature range of a bathroom during a hot shower, both being reasonable environments where a BPO-containing product may be stored by a consumer. The results of these tests clearly show that benzene can leak outside of unopened BPO-containing acne treatment products at concerningly high levels.”
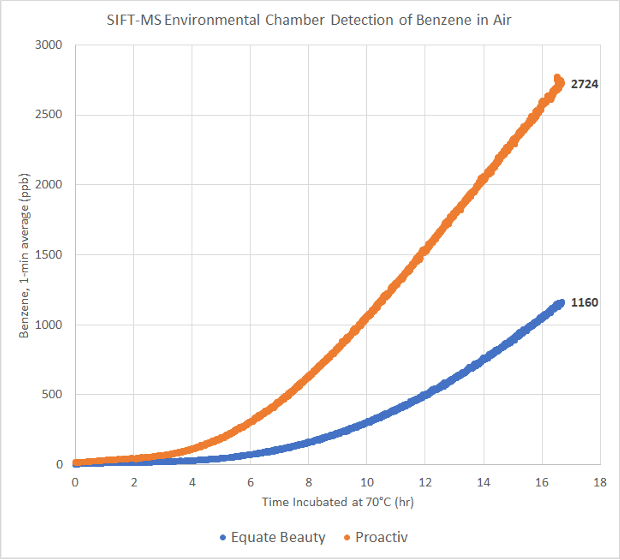
The above is Figure 7 from Valisure’s Petition and shows SIFT-MS analysis of benzene in the air within a 528L environmental chamber while a product is incubated at 70°C for 16.7 hours. Detected benzene is displayed in ppb and as a moving average of 1 minute of detection (20 detections occurring at 3-second intervals in real-time). Both products were incubated without modification from original packaging; notably their caps were not opened.
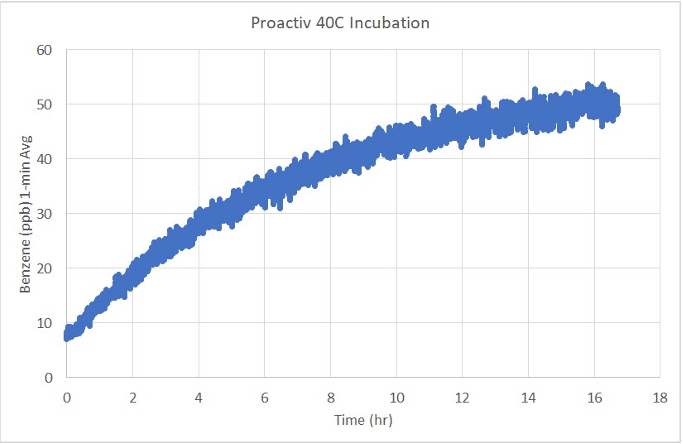
Figure 8 from the Petition shows SIFT-MS analysis of benzene in the air within a 528L environmental chamber while a product is incubated at 40°C for 16.7 hours. Detected benzene is displayed in ppb and as a moving average of 1 minute of detection (20 detections occurring at 3-second intervals in real-time). Product was incubated without modification from original packaging; notably the cap was not opened.
Navigating Consumer Concerns
It is important to point out that the benzene levels in these BPO-containing products do not stay the same. According to Light “The level of benzene can increase, because the benzoyl peroxide itself is unstable and makes more benzene over time, especially with any exposure to elevated temperatures. Even if a product is clean when purchased, it can form high levels of benzene over time even sitting in a medicine cabinet.” He goes on to state “This is a complex issue, and it is not a simple issue of benzene contamination from raw materials that we saw in sunscreens and hand sanitizers. This is the fundamental instability of the molecule, and that’s why we do these stability studies, which are regulatory requirements and have published them in this FDA Citizen’s Petition.”
As per the latest guidelines from the Journal of the American Academy of Dermatology (AAD) on acne vulgaris care and management, benzoyl peroxide is strongly recommended for acne treatment. However, considering the recent findings, it remains uncertain whether these guidelines will necessitate reassessment in the near future.
With many consumers now facing uncertainty about their acne products, Light recommends that clinicians and their patients look to other forms of acne treatment that do not contain BPO.
Valisure also tested salicylic acid-containing products, which the AAD conditionally recommends for acne treatment, but found no benzene contamination in them.
“Data in the Petition suggests that only BPO-containing acne treatment products have this issue of forming high levels of benzene, and that other acne treatment products tested by Valisure, such as those containing salicylic acid or adapalene, do not appear to have this problem.”
Dr. Bunick adds, “Our patients entrust us with their well-being, and it is our responsibility to ensure that the products we use and recommend in our practices meet the highest standards of safety and quality.”
Moving Forward
The aim of Valisure’s Citizen’s Petition is not only to recall and suspend the sale of these products with dangerously high levels of benzene but also to reform the way BPO products are formulated in the future by improving the quality control processes and guidance for drug manufacturers and distributors.
One of their requests is for independent chemical testing and verification of medications. They point out that European regulators have recognized the importance of independent testing and since established a system of over 70 Official Medicines Control Laboratories.
The Petition also refers to their former study which examined ranitidine (brand name Zantac). They found that the molecular instability which caused the formation of a potent carcinogen, N-nitrosodimethylamine, (NDMA) resulted in the full-market withdrawal of the drug with approximately 1 time the regulatory limit for NDMA at 70°C for two weeks. They validate their current request by stating that these BPO-containing products can form over 800 times the regulatory limit for benzene at the lower temperature of 50°C for two weeks.
In response to Valisure’s Citizen’s Petition, the FDA states it will work to verify the accuracy of Valisure’s data before acting on the petition. Jeremy Khan, a spokesperson for the agency, has stated “The agency will continue to provide updates to the public regarding benzene in drug products, as appropriate,” adding that companies are required to ensure the safety of their products.
Benzene Discussions at AAD
The news of benzene in BPO-containing products was among the many topics covered at the recent American Academy of Dermatology (AAD) Annual Meeting which took place in San Diego, California from Mar. 8-12, 2024. Dr. Bunick, who was present at the conference, addressed attendees about the recent discovery by Valisure and provided updates on the issue.
Some have criticized Valisure’s findings, raising questions about their stability testing at such high temperatures. Valisure has since published a news release outlining the importance of stability testing and why it is conducted. The statement notes, “The purpose of stability studies is not to copy the conditions of an average consumer, rather, the purpose is to ensure the product is safe to use for the entire products lifecycle, from manufacturing through distribution to its expiration date, often a total of 3 years for pharmaceutical products. Products must be stable during this entire time and through all the possible “bumps” along the road. It is common to condense the total stability of a product intended for multiple years of use by performing “accelerated” stability studies that elevate the temperature for a few months. For example, according to publicly available calculators, 3 years of room temperature stability is equal to about 169 days at 50°C (122°F). Valisure broadly observed dramatically high levels of benzene in only 18 days at 50°C.”
The release also explains the significance of fully understanding the “Day 0” results for the 66 BPO-containing acne products that were tested. Day 0 levels of benzene is an indication of the benzene levels detected in these products at room temperature before the products were even incubated. “Of the 66 benzoyl peroxide products tested, 10 products had over 10 ppm of benzene and 19 products had over 2 ppm, which means many products already had unacceptably high benzene levels when Valisure purchased them.”
Referring to their testing approach, Valisure’s statement further outlines the FDA’s preferences on ‘Storage Conditions’ in their “Expiration Dating and Stability Testing for Human Drug Products” guide which states “Stability studies should be conducted on product stored under normal storage conditions or, preferably, under exaggerated conditions.”
During his presentation, Dr. Bunick also made reference to the recent statement by the U.S. Pharmacopeia (USP) of Mar. 8, 2024, which notes that the presence of unsafe levels of benzene should be taken seriously, but that “If changes are made to a USP method, complete validation data is necessary to demonstrate that a product meets USP standards.” According to Dr. Bunick, “The most important line in this entire statement is the next one: ‘It is important to note that USP standards are applicable during the entire shelf life of the product, not just at the time of release.’“ The problem with the USP statement is that the FDA already rejected their methods as not being good enough, and you wouldn’t know that unless you read the FDA’s statement.” He goes on to explain that the FDA had previously rejected USP’s method of gas chromatography- flame ionization detection (GC-FID) for benzene analysis in 2020 as it is less accurate than their preferred method of gas chromatography-mass spectrometry (GC-MS) which is what Valisure utilized in their study.
Speaking of the concern surrounding Valisure’s testing under elevated temperatures, Dr. Bunick states, “There’s been concerns that superficially when you look at the petition, that it’s all about elevated temperatures. That’s not true. Benzene was also found extensively at room temperature on the shelf/off the shelf. The focus of the petition is stability studies.” He reminded attendees that the benzene in BPO-containing acne products is not as a result of contamination, but instead a problem with BPO degradation, adding that the FDA allows up to 2 ppm of benzene only if it is absolutely necessary to manufacture the product. Because there are so many other treatments available for the treatment of acne, it cannot be said these products with such unsafe levels of benzene are ‘absolutely necessary.’
Dr. Bunick went on to share new data with the audience which demonstrated that of five BPO-containing products that were tested at 37°C (human skin temperature) one had more than 10 ppm of benzene, and all five had over 2 ppm of benzene.
Moving forward with this current information may prove challenging for dermatologists and their patients as they evaluate their next steps. Dr. Bunick provided his fellow colleagues with some advice and recommendations, “With Valisure’s agreement, that for those providers and patients who wish to continue benzoyl peroxide-containing products, that a proportional response at this time would be to keep the product refrigerated at all times, renew the medicine every 3 to 6 months, and avoid heated storage. This will not necessarily eliminate all benzene but should slow its decomposition. This measure gives the medical community a chance to await further investigation and recommendations.”
Updated: March 18, 2024
Sources:
- Detectable Levels of Benzene Noted in Some Sunscreen Batches
- Valisure Detects Benzene in Benzoyl Peroxide
- BREAKING NEWS: Benzene Found in Various Acne Products; Valisure Files Petition With FDA to Recall Treatments
- Valisure Detects Benzene in Sunscreen
- Chemical Linked to Cancer Found in Acne Creams Including Proactiv, Clearasil
- P&G Issues Voluntary Recall of Specific Old Spice and Secret Aerosol Spray Antiperspirants and Old Spice Below Deck Aerosol Spray Products Due to Detection of Benzene
- Unilever Issues Voluntary U.S. Recall of Select Dry Shampoos Due to Potential Presence of Benzene
- Yale expert explains dangers in recalled dry hair shampoos
- Suave antiperspirants are recalled because benzene was found in some samples
- Timeline of consumer product recalls due to benzene
- Dermatological Dilemma: Unmasking the Benzene Menace in Our Products
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products
- Valisure’s David Light Unpacks Benzene Findings, Stability Testing, and Discourse
- Guidelines of care for the management of acne vulgaris – Journal of the American Academy of Dermatology (jaad.org)
Expiration Dating and Stability Testing for Human Drug Products
















