As the most common form of eczema, atopic dermatitis (AD) affects over 9.6 million children and about 16.5 million adults in the United States.
The enduring prevalence of this inflammatory skin condition resulted in the development of topical corticosteroids (TCS) and calcineurin inhibitors (TCI). And for decades, these were considered the mainstay in AD treatment. Not just because they are considered effective but because they were also the only impactful options.
However adequate these topical corticosteroids and emollients are, there remain concerns surrounding their continuous, long-term use. Side effects previously reported include localized skin atrophy, telangiectasia, striae, perioral dermatitis, and acne.
Fortunately, recent years have seen a dramatic increase in research concerning AD, which has led to an overall improved understanding of the condition. Although there is much to be uncovered, several studies have succeeded in shedding light on some of the most important underlying factors that come into play.
As a result, we have now entered a revolutionary era of medicine. The introduction of JAK inhibitors, such as ruxolitinib and abrocitinib, are considered breakthroughs in the treatment landscape, not only for AD but for a variety of other conditions as well.
Ruxolitinib, the first JAK inhibitor cream for the treatment of AD, was approved by the U.S. Food and Drug Administration (FDA) in September 2021. It is targeted for the short-term and non-continuous chronic treatment of mild to moderate AD in non-immunocompromised patients over 12 years of age. Based on data from the TRuE-AD (Topical Ruxolitinib Evaluation in Atopic Dermatitis) clinical trial program, patients experienced significantly clearer skin and itch reduction. To add to its treatment efficacy, a study recently published in the Journal of the European Academy of Dermatology and Venereology found ruxolitinib cream demonstrated a rapid improvement in itch in patients with mild to moderate AD over an eight-week period.
Dupilumab is the first biologic medication approved by the FDA for adults and children six years and up with moderate to severe atopic dermatitis. Dupilumab has been welcomed by dermatologists and their patients.
"Until today, treatment options in the U.S. for infants and children under the age of 6 suffering from moderate-to-severe atopic dermatitis have been limited to topical steroids – which may be associated with significant safety risks when used long-term."
Naimish Patel, M.D, Senior Vice President, Head of Global Development, Immunology and Inflammation at Sanofi.
Several other clinical trials are planned or currently underway for other biological medications for AD.
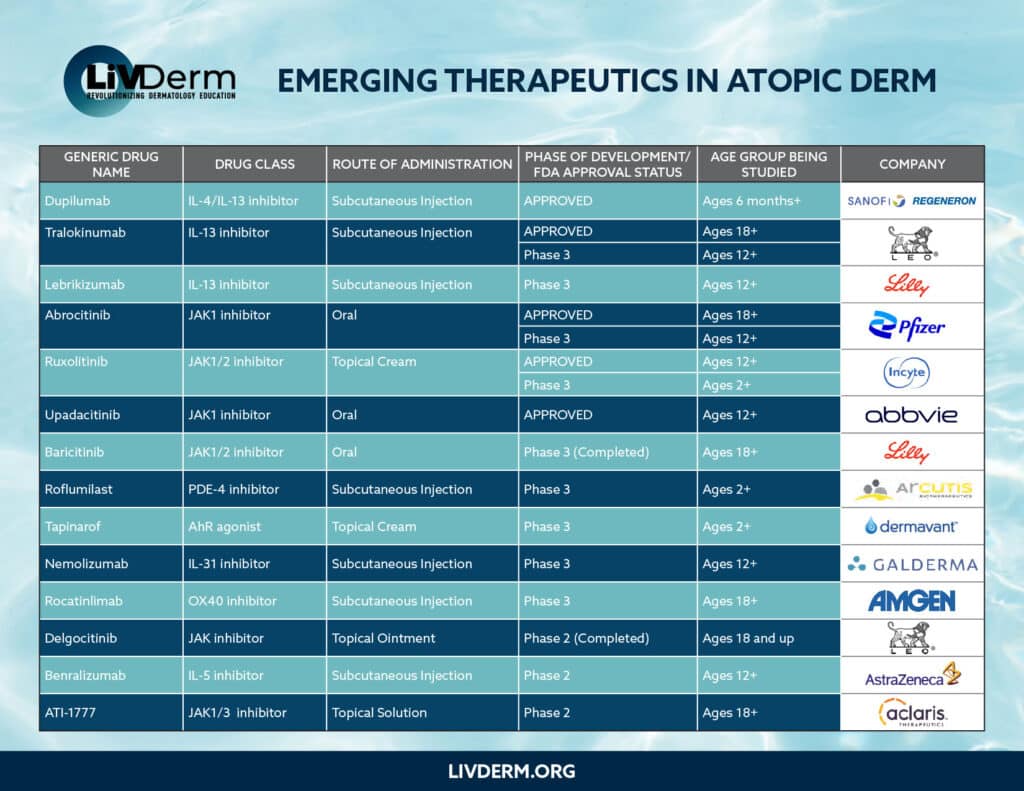
These novel and emerging treatments are a much-needed advancement in the industry. Not only do they provide additional options to the historical TCSs and TCIs, but many are more effective and offer longer-lasting results. As researchers continue with their trials and investigations, we can be sure of even more promising treatments and therapies in the coming years.
Visit LiVDerm’s Atopic Dermatitis Digital Education Hub for more information and resources.
















